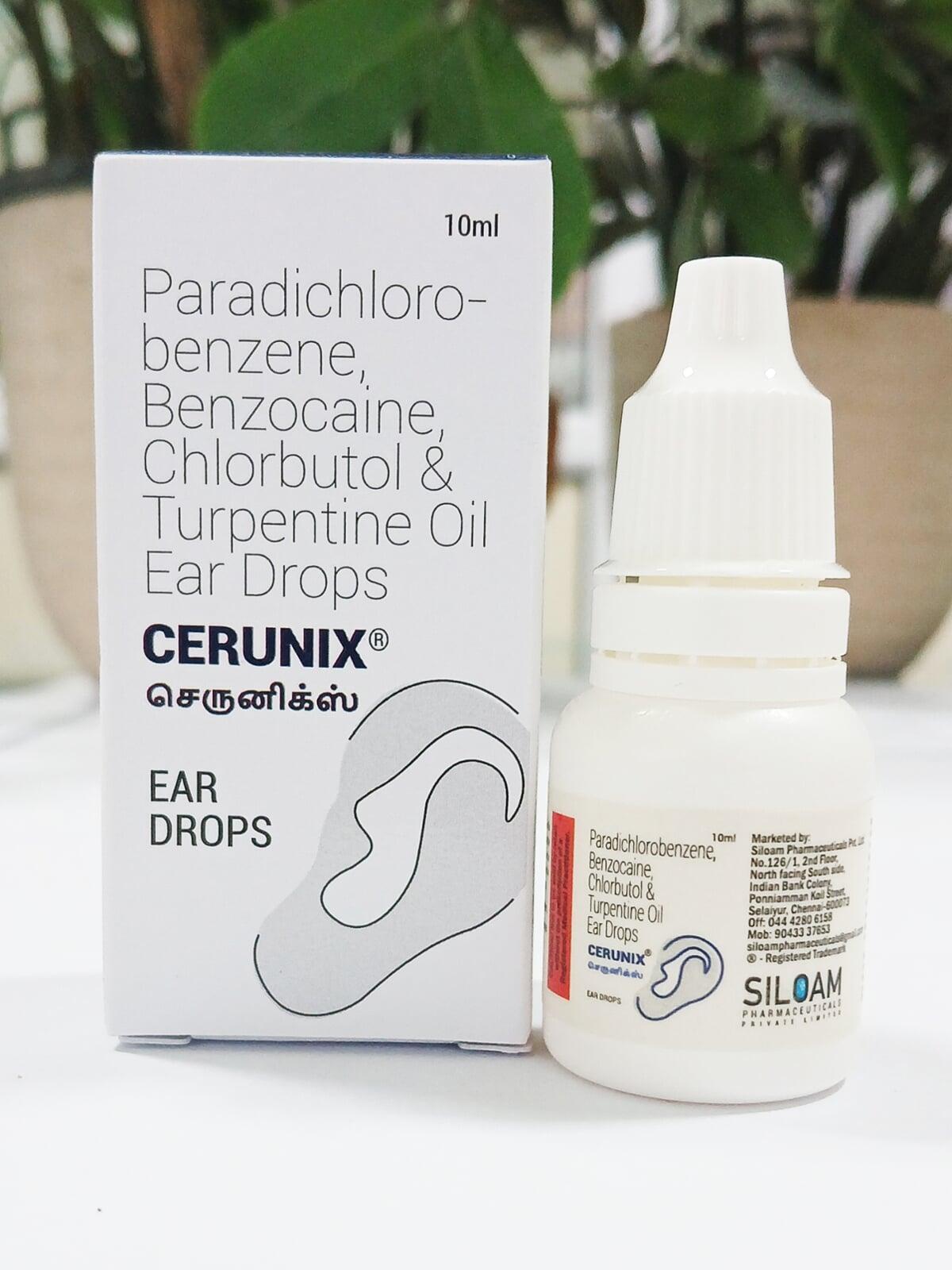
CERUNIX EAR DROPS
COMPOSITION:
| Each drop contains: | |
| Paradichlorobenzene | 2% w/v |
| Benzocaine | 2.7% w/v |
| Chlorbutol | 5% w/v |
| Turpentine oil | 15% w/v |
KEEP ALL MEDICINES OUT OF THE REACH OF CHILDREN.
- Temporary relief of ear pain associated with ear infections, inflammation, or minor injuries.
- Prevention and treatment of ear infections caused by bacteria and fungi.
- Relief from itching and discomfort in the ear canal caused by minor irritations.
- Removal of earwax and softening of impacted earwax (though not a primary function).
- Tilt the head sideways and instil the drops into the ear canal.
- Keep the head tilted for 2–3 minutes to allow absorption.
- Do not insert cotton swabs or objects into the ear after application.
- Do not use in cases of a perforated eardrum (hole in the eardrum), as it may lead to systemic absorption and further complications.
- Use with caution in individuals with sensitive skin or allergies to any of the ingredients.
- Avoid contact with the eyes, as the ingredients may cause irritation.
- If pain persists or ear discharge occurs, seek medical attention as it may indicate a more serious infection or condition.
- Not for prolonged use: Extended use of ear drops may result in potential side effects, including irritation or allergic reactions.
- Turpentine oil may cause local irritation if used too frequently.
- Avoid using in combination with other ear products without consulting a healthcare provider.
- Perforated eardrum (e.g., due to trauma or infection) – may cause systemic absorption and harm.
- Hypersensitivity to any component of the ear drops (Paradichlorobenzene, Benzocaine, Chlorbutol, or Turpentine Oil).
- Active ear infection with drainage – avoid use until the ear is properly evaluated by a healthcare professional.
- No significant systemic drug interactions are expected when the drops are used as directed since the ingredients are locally acting.
- Caution is advised if using other topical products in or around the ear, especially those containing strong irritants or antiseptics.
- Avoid use with other local anaesthetics (such as lidocaine) to prevent excessive numbing or irritation in the ear.
- Pregnancy: The safety of this combination during pregnancy is not well-established. Use only if clearly needed, and consult a healthcare provider before use.
- Lactation: Benzocaine, Turpentine Oil, and Paradichlorobenzene are minimally absorbed systemically when applied topically, but consult a doctor before use, especially if breastfeeding.
- Local irritation or mild burning sensation in the ear.
- Itching or redness around the ear or ear canal.
- Temporary numbness or tingling in the ear (due to Benzocaine).
- Allergic reactions, including rash, swelling, or difficulty breathing (rare).
- Persistent pain, discharge, or worsening symptoms may indicate a more severe underlying condition such as an ear infection or perforated eardrum.
- Overdose is unlikely with proper use, as the ear drops are applied locally and not absorbed in significant quantities.
- Symptoms of overdose could include excessive irritation or burning sensation in the ear, or severe allergic reactions if an individual is sensitive to any of the ingredients.
- If accidental ingestion or misuse occurs, seek immediate medical attention.
- Wash the ear thoroughly with warm water to remove excess product.
- Supportive treatment should be provided for any severe reactions, including antihistamines for allergic reactions.
- Store the ear drops at room temperature (15–25°C).
- Keep the bottle tightly closed and out of reach of children.
- Avoid exposure to direct sunlight or extreme temperatures.
- Do not refrigerate or freeze the product.
- Check the expiry date and discard the product once it has expired.
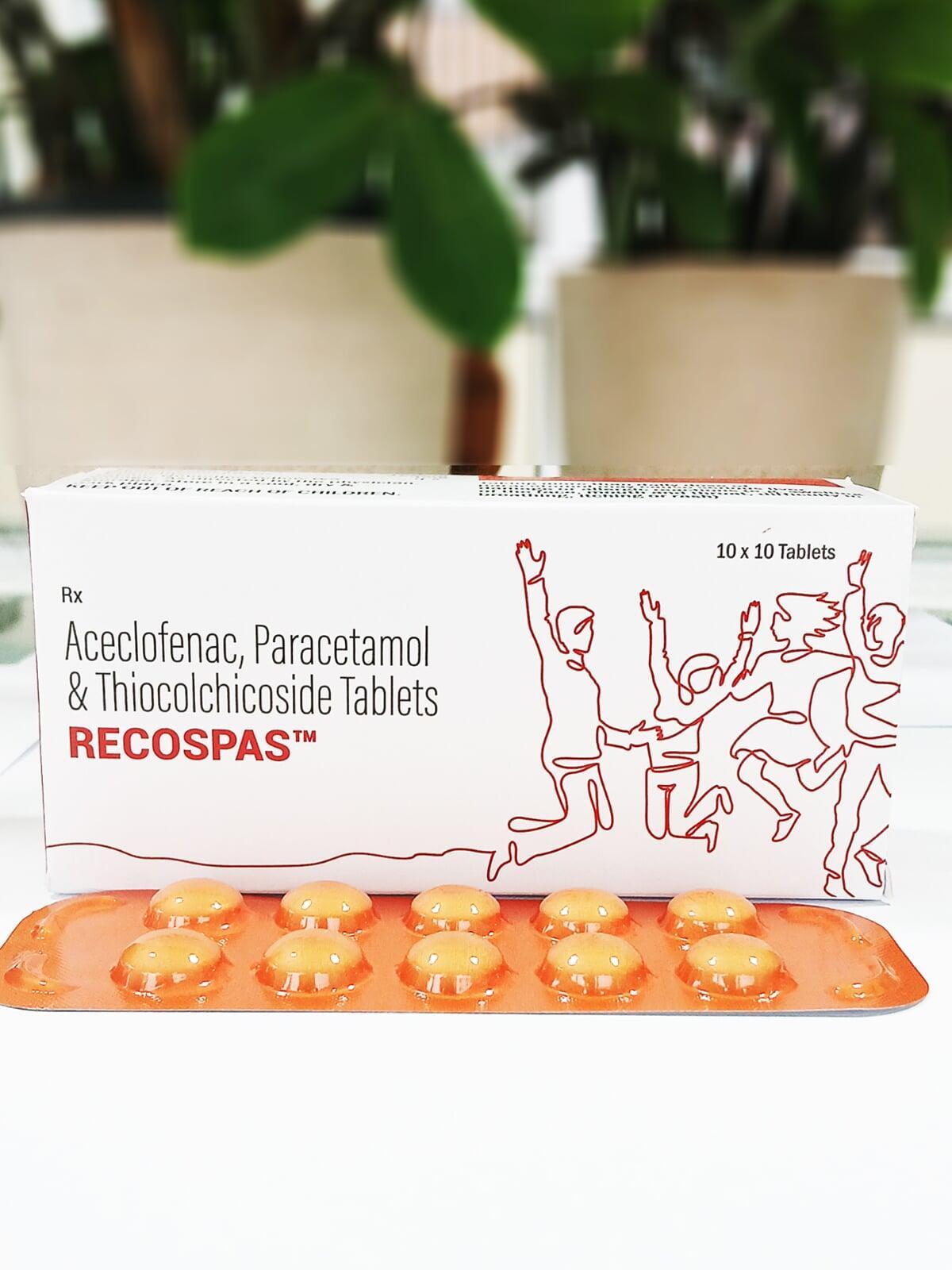
RECOSPAS TABLET
COMPOSITION:
| Each film coated tablet contains: | |
| Aceclofenac IP | 100 mg |
| Paracetamol IP | 325 mg |
| Thiocolchicoside IP | 4 mg |
| Excipients | q.s. |
KEEP ALL MEDICINES OUT OF THE REACH OF CHILDREN.
Aceclofenac:
A non-steroidal anti-inflammatory drug (NSAID) that inhibits cyclooxygenase (COX-1 and COX-2) enzymes, leading to decreased prostaglandin synthesis. It reduces pain and inflammation.
Paracetamol: An analgesic and antipyretic. It works primarily in the CNS, inhibiting COX enzymes and elevating the pain threshold. It has minimal anti-inflammatory activity.
Thiocolchicoside: A muscle relaxant with anti-inflammatory and analgesic properties. It acts on the GABA-A receptors and glycine receptors in the CNS, helping reduce painful muscle spasms. INDICATIONS Recospas
Tablet is indicated for the relief of:
- Acute painful musculoskeletal conditions
- Muscle spasms
- Back pain, neck pain, and other orthopaedic and post-traumatic pain states
- Pain associated with sprains, strains, and sports injuries
Recospas Tablet is indicated for the relief of:
- Acute painful musculoskeletal conditions
- Muscle spasms
- Back pain, neck pain, and other orthopaedic and post-traumatic pain states
- Pain associated with sprains, strains, and sports injuries
Adults: One tablet twice daily after food or as directed by the physician.
Not recommended for children below 18 years unless prescribed by a physician.
- Use with caution in patients with hepatic or renal impairment.
- Avoid alcohol consumption due to increased risk of liver toxicity (due to Paracetamol).
- Monitor liver function if used for a prolonged period.
- Risk of gastrointestinal bleeding or ulceration (due to Aceclofenac).
- Can cause drowsiness (due to Thiocolchicoside); avoid operating machinery or driving.
- Caution in elderly patients as they are more sensitive to NSAIDs.
- Not recommended, especially in the third trimester.
- Thiocolchicoside has shown teratogenic effects in animal studies.
- Paracetamol is generally considered safe but not when combined with other agents unless advised by a physician.
- Gastrointestinal: Nausea, vomiting, dyspepsia, diarrhoea, gastritis.
- Neurological: Drowsiness, dizziness.
- Hepatic: Elevated liver enzymes.
- Dermatologic: Rash, allergic reactions.
- Others: Renal dysfunction (rare), hypersensitivity reactions.
- Paracetamol overdose can cause severe hepatotoxicity.
- Aceclofenac overdose may cause GI bleeding, renal dysfunction, CNS depression.
- Thiocolchicoside overdose may cause seizures.
- Supportive and symptomatic treatment.
- Gastric lavage if within 1 hour of ingestion.
- Activated charcoal if appropriate.
- N-acetylcysteine for paracetamol toxicity.
- Monitor liver and renal function closely
- Store in a cool, dry place.
- Protect from direct sunlight and moisture.
- Keep out of reach of children.
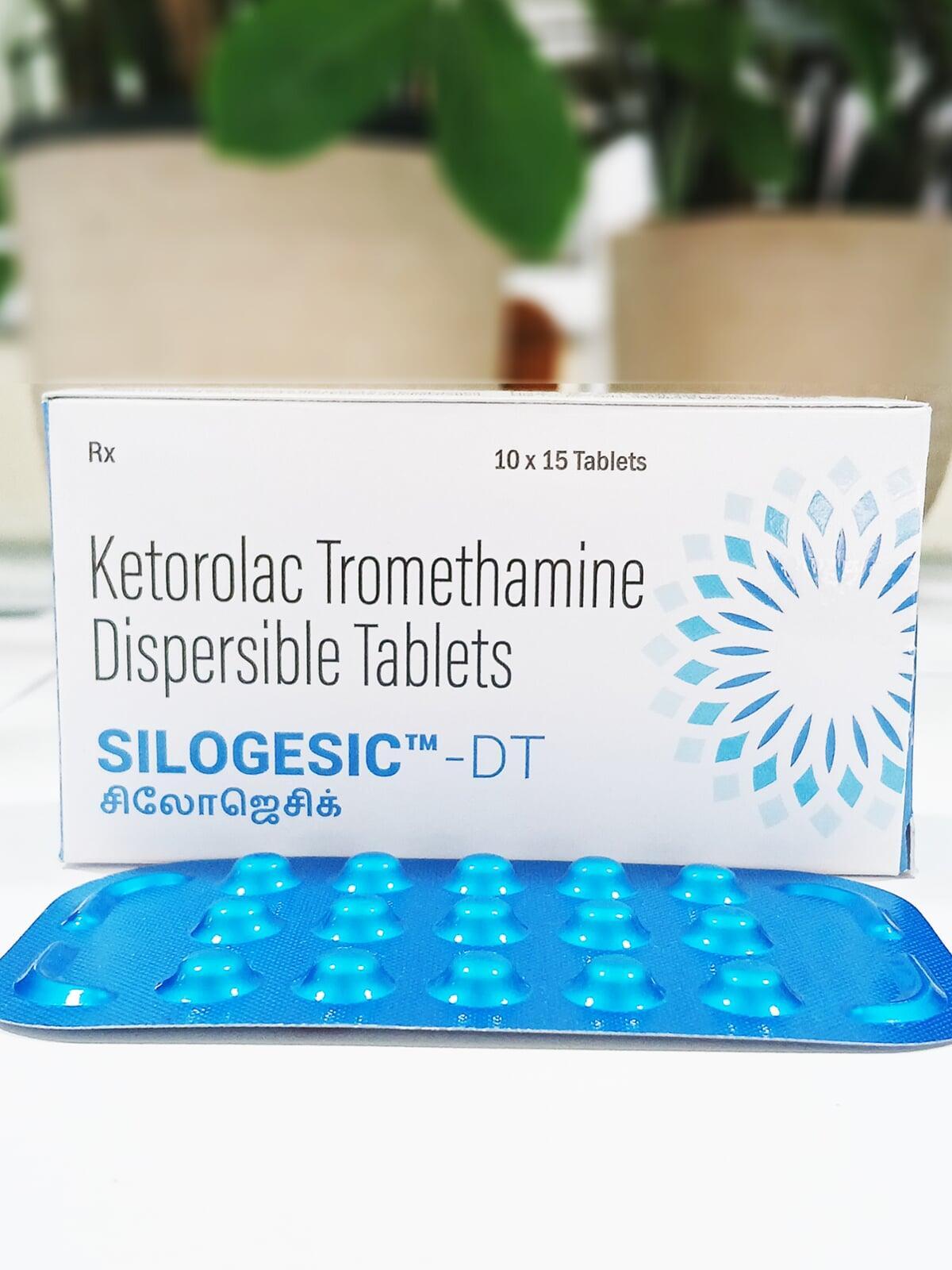
SILOGESIC DT
COMPOSITION:
| Each uncoated dispersible tablet contains: | |
| Ketorolac Tromethamine I.P. | 10 mg |
| Excipients | q.s. |
KEEP ALL MEDICINES OUT OF THE REACH OF CHILDREN.
Ketorolac Tromethamine is a potent non-steroidal anti-inflammatory drug (NSAID) of the pyrrolo-pyrrole group.
∙Mechanism of Action: Inhibits cyclooxygenase (COX-1 and COX-2) enzymes, thereby reducing prostaglandin synthesis involved in pain, inflammation, and fever.
Pharmacokinetics
∙Onset of Action: Within 30–60 minutes.
∙Half-life: ~5–6 hours.
∙Metabolism: Hepatic. ∙Excretion: Primarily renal.
Silogesic Tablet (Ketorolac 10 mg) is indicated for:
Short-term management of moderate to severe acute pain, including:
- Postoperative pain
- Musculoskeletal pain
- Renal colic
- Dental pain
- Trauma or injury-related pain
Note: It is not indicated for chronic pain or minor aches due to its strong GI and renal side effect profile.
Adults (Oral):
∙10 mg every 4–6 hours as needed.
∙Maximum daily dose: 40 mg/day.
∙Duration of use: Not more than 5 days (oral therapy), due to risk of adverse effects.
Paediatric: Not recommended for children under 16 years unless prescribed.
- Use the lowest effective dose for the shortest duration.
- Not intended for long-term or chronic pain treatment.
- Adequate hydration must be maintained to minimize renal risk.
- Increased risk of gastrointestinal bleeding, ulceration, or perforation, especially in elderly patients.
- Can cause renal impairment, particularly in volume-depleted patients.
- May elevate blood pressure and should be used cautiously in hypertensive patients.
- Can impair platelet aggregation and prolong bleeding time.
- Hypersensitivity to ketorolac or other NSAIDs.
- Active peptic ulcer disease or recent GI bleeding/perforation.
- Renal impairment or dehydration.
- History of asthma, urticaria, or allergic-type reactions after taking NSAIDs.
- Concurrent use with aspirin or other NSAIDs.
- Perioperative pain management in CABG surgery.
- Pregnancy (third trimester) and lactation.
- Anticoagulants/Antiplatelets: Increased risk of bleeding.
- ACE inhibitors/ARBs/Diuretics: Increased risk of nephrotoxicity.
- Lithium: Increased serum lithium levels and toxicity.
- Methotrexate: Enhanced toxicity.
- SSRIs/SNRIs: Increased risk of GI bleeding.
- Category C (first and second trimester), Category D (third trimester).
- Contraindicated in third trimester due to risk of premature closure of the ductus arteriosus and prolonged labour.
- Avoid in breastfeeding unless benefit outweighs risk.
- Common: Nausea, abdominal pain, dyspepsia, headache, dizziness.
- Serious: GI bleeding, peptic ulceration, renal dysfunction, hypertension, hypersensitivity reactions, bronchospasm.
- Nausea, vomiting, abdominal pain, GI bleeding, drowsiness, renal failure, metabolic acidosis.
- Supportive and symptomatic treatment.
- Activated charcoal if within 1 hour of ingestion.
- Monitor renal and hepatic function, and blood counts.
- No specific antidote available.
- Store below 25°C in a cool, dry place.
- Protect from light and moisture.
- Keep out of reach of children
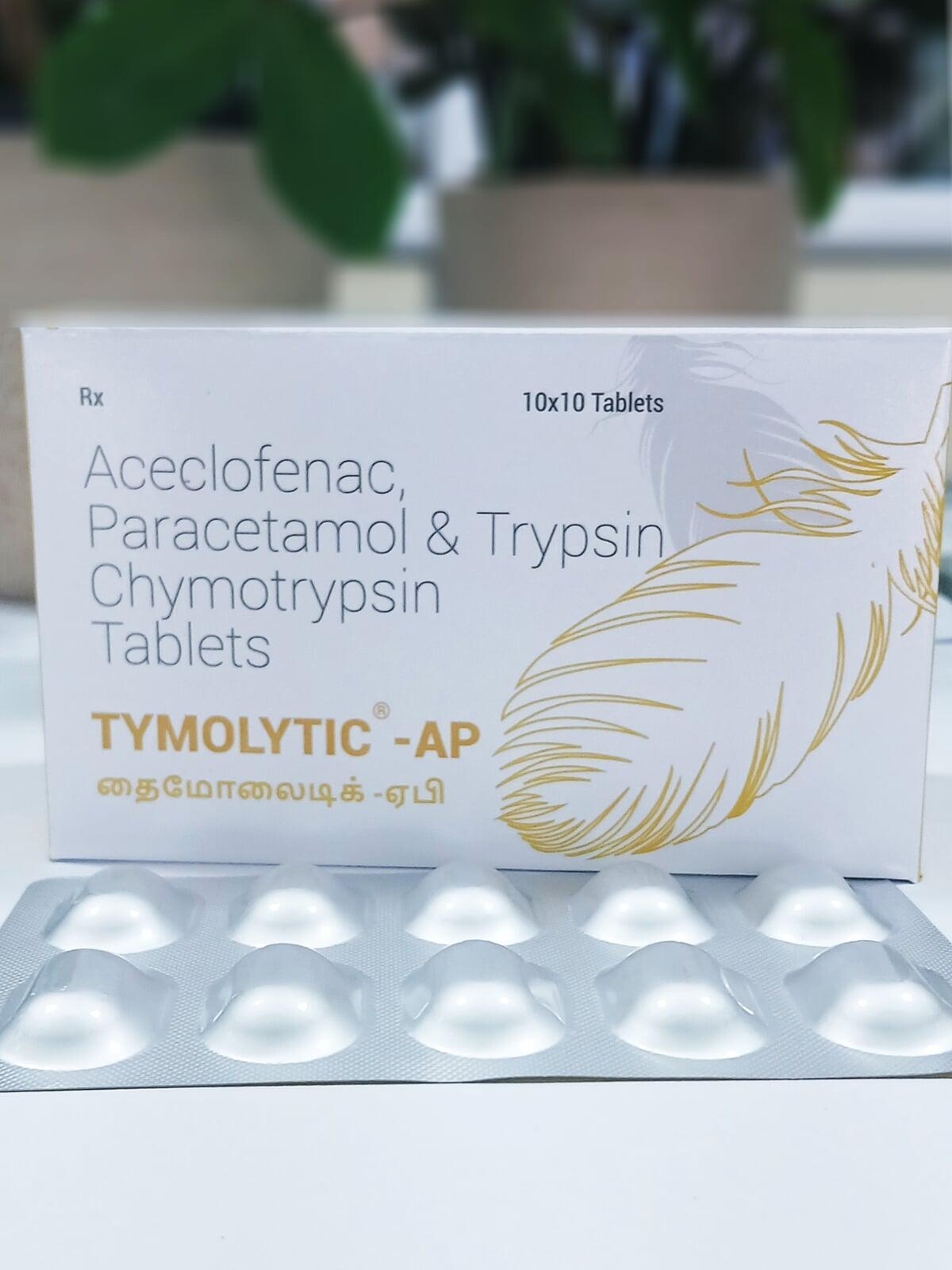
TYMOLYTIC AP TABLET
COMPOSITION:
| Each film coated tablet contains: | |
| Aceclofenac IP | 100 mg |
| Paracetamol IP | 325 mg |
| 50000 Armour units of enzymatic activity | |
| (supplied by a purified concentrate which has specific Trypsin Chymotrypsin activity in a ratio of approximately 6:1) | |
| Excipients | q.s. |
KEEP ALL MEDICINES OUT OF THE REACH OF CHILDREN.
Trypsin + Chymotrypsin (Proteolytic Enzyme Complex)
∙Derived from pancreatic enzymes, these proteolytic enzymes enhance tissue repair by breaking down fibrin, proteins, and inflammatory debris.
∙Anti-inflammatory by reducing edema and inflammation at injury sites.
∙Trypsin and Chymotrypsin together increase microcirculation and support healing.
Aceclofenac
∙A non-steroidal anti-inflammatory drug (NSAID).
∙Inhibits cyclooxygenase (COX-2 > COX-1), thereby reducing prostaglandin synthesis.
∙Provides analgesic, anti-inflammatory, and antipyretic effects.
Paracetamol (Acetaminophen)
∙A centrally acting analgesic and antipyretic.
∙Inhibits prostaglandin synthesis in CNS.
∙Less anti-inflammatory activity than NSAIDs, but excellent for pain and fever.
Synergy:
∙Aceclofenac + Paracetamol relieve pain, inflammation, and fever,
∙Trypsin-Chymotrypsin accelerates healing and edema reduction.
- Postoperative inflammation and pain
- Musculoskeletal pain (sprains, strains, tendonitis)
- Orthopaedic trauma or sports injuries
- Dental surgeries and infections ENT surgeries (tonsillectomy, sinusitis-related edema)
- Soft tissue inflammation, hematomas, and contusions
- Osteoarthritis, spondylitis, and rheumatic conditions
Adults:
1 tablet twice daily, preferably 1 hour before meals or as advised by a physician
Adjust dose based on clinical condition and physician recommendation.
Children:
Not typically recommended. Use under medical supervision only.
- Use with caution in elderly, renal impairment, or dehydrated individuals.
- Not recommended for long-term use without medical supervision.
- May cause gastrointestinal irritation, especially with prolonged use.
- Use cautiously in hypertensive or cardiac patients due to fluid retention risk (NSAID component).
- Liver enzyme monitoring is advised for long-term use due to paracetamol.
- Hypersensitivity to any of the ingredients.
- Active or history of gastric ulcers or GI bleeding.
- Severe hepatic or renal impairment.
- Bleeding disorders (due to proteolytic enzymes).
- Pregnancy (3rd trimester) due to NSAID component.
- Anticoagulants (e.g., warfarin) – Increased bleeding risk.
- Methotrexate, lithium – Enhanced toxicity.
- Alcohol – Increased hepatotoxicity with paracetamol.
- Other NSAIDs or corticosteroids – Additive GI toxicity.
- CE inhibitors/Diuretics – Reduced efficacy, increased renal risk.
- Avoid in 3rd trimester due to potential for premature ductus arteriosus closure (NSAIDs).
- Use in 1st and 2nd trimester only if clearly necessary.
- Not recommended during lactation unless benefits outweigh risk.
- Gastrointestinal: Nausea, dyspepsia, epigastric pain, ulcers.
- Renal: Elevated serum creatinine, reduced GFR (rare).
- Hepatic: Elevated liver enzymes (with paracetamol).
- Allergic: Rash, itching, rarely angioedema or anaphylaxis.
- Enzymes: Mild nausea, altered taste, rarely allergic reactions.
- Aceclofenac: GI bleeding, renal damage.
- Paracetamol: Hepatotoxicity, especially >4 g/day.
- Trypsin-Chymotrypsin: Generally safe, but may cause nausea or GI upset in excess.
- Symptomatic support.
- For paracetamol overdose: N-Acetylcysteine (NAC) is the antidote.
- Monitor LFTs, renal function, and electrolytes.
- Store at 15–25°C in a cool, dry place.
- Protect from light and moisture.
- Keep out of reach of children.
TYMOLYTIC PLUS TABLET

COMPOSITION:
| Each enteric coated tablet contains: | |
| 50000 Armour units of enzymatic activity | |
| (supplied by a purified concentration which has specific Trypsin and Chymotrypsin activity in a ratio of approximately 6:1) | |
| Diclofenac Potassium IP | 50 mg |
| Excipients | q.s. |
KEEP ALL MEDICINES OUT OF THE REACH OF CHILDREN.
Trypsin + Chymotrypsin (6:1)
∙Proteolytic enzymes derived from the pancreas.
∙Work by breaking down inflammatory proteins and fibrin, reducing swelling, edema, and enhancing microcirculation at the inflammation site.
∙Improve wound healing, resolve post-traumatic and surgical edema, and support tissue regeneration.
Diclofenac Potassium
∙A fast-acting NSAID with analgesic, antipyretic, and anti-inflammatory effects.
∙Inhibits cyclooxygenase (COX-1 and COX-2), decreasing prostaglandin synthesis which reduces inflammation and pain.
∙Potassium salt form ensures rapid absorption and onset of action.
Synergy:
Diclofenac provides quick pain relief, while Trypsin-Chymotrypsin supports tissue repair and swelling reduction, making it ideal for injury and post-op recovery.
Tymolytic Plus is indicated for:
∙Episiotomy
∙Musculoskeletal injuries (sprains, strains, contusions)
∙Postoperative inflammation and edema
∙Orthopaedic trauma and joint pain
∙Arthritis, spondylitis, bursitis, and tendinitis
∙ENT surgeries, sinusitis, and soft tissue inflammation
∙Sports injuries and inflammatory swelling
Adults:
1 tablet twice daily, preferably 1 hour before meals or as advised by a physician.
Children:
Not recommended unless prescribed by a healthcare professional.
- Use with caution in elderly, renal impairment, or gastric ulcer history.
- Ensure adequate hydration during treatment.
- Long-term use may lead to GI, hepatic, or renal side effects.
- Avoid alcohol during therapy due to enhanced GI and liver risks.
- May mask symptoms of infection; use cautiously in infected patients.
- Hypersensitivity to diclofenac, proteolytic enzymes, or excipients.
- Active peptic ulcer, GI bleeding, or perforation history.
- Severe hepatic, renal, or cardiac impairment.
- Known bleeding disorders. ∙Asthma, urticaria, or allergic reaction triggered by NSAIDs.
- Anticoagulants (e.g., warfarin) – Increased risk of bleeding.
- Other NSAIDs or corticosteroids – Enhanced GI toxicity.
- Diuretics, ACE inhibitors – Increased renal risk.
- Methotrexate, lithium – Enhanced toxicity.
- Antibiotics (e.g., amoxicillin) – Absorption may be enhanced by bromelain.
- Not recommended in the 3rd trimester – risk of premature ductus arteriosus closure.
- Use with caution in 1st and 2nd trimesters if benefits outweigh risks.
- Avoid in lactating women, unless prescribed.
- GI: Nausea, vomiting, abdominal pain, dyspepsia, ulcers, bleeding.
- Hepatic: Elevated liver enzymes.
- Renal: Nephrotoxicity (rare).
- CNS: Headache, dizziness.
- Hypersensitivity: Rash, itching, urticaria.
- Enzymatic: Rare GI upset, altered taste, allergic reactions.
- Diclofenac: GI bleeding, renal failure, hypotension, CNS depression.
- Trypsin-Chymotrypsin: Generally well-tolerated; may cause GI upset in high doses.
- Supportive and symptomatic treatment.
- Monitor liver and renal function.
- Gastric lavage and activated charcoal if early.
- Store in a cool, dry place below 25°C.
- Protect from light and moisture.
- Keep out of reach of children.
TYMOLYTIC NANOGEL
COMPOSITION:
| Each gm contains: | |
| Diclofenac Diethylamine IP | 4.64% w/v |
| (equivalent to Diclofenac Sodium) | 4.00% w/v |
| In Methyl Salicylate Gel Base |
KEEP ALL MEDICINES OUT OF THE REACH OF CHILDREN.
- Musculoskeletal pain (sprains, strains, and sports injuries)
- Arthritis (osteoarthritis, rheumatoid arthritis)
- Backache, neck pain, shoulder stiffness
- Tendinitis, bursitis, myositis
- Soft tissue injuries and localized inflammation
- Apply a small quantity (2–4 g) to the affected area 3–4 times daily.
- Gently massage until fully absorbed.
- Do not use on broken or irritated skin.
- Wash hands after application unless hands are the treated area.
- For external use only.
- Avoid contact with eyes, mucous membranes, or open wounds.
- Do not apply with occlusive bandages or heat packs.
- Prolonged use may lead to local irritation.
- Do not use on infected skin lesions or eczema.
- if irritation or allergic reaction occurs, discontinue use.
- Hypersensitivity to diclofenac, salicylates, or other NSAIDs.
- Broken or inflamed skin. ∙
- Third trimester of pregnancy.
- Minimal systemic absorption — low potential for interactions.
- Avoid use with other topical NSAIDs or salicylates to prevent cumulative irritation.
- Avoid during third trimester due to risk of premature ductus arteriosus closure.
- Use in first and second trimesters only if clearly needed.
- Avoid during lactation on or near the chest area.
- Local: Rash, redness, pruritus, burning sensation.
- Systemic (rare): Headache, nausea, dizziness (with overuse or prolonged use).
- Topical overdose is rare.
- Symptoms: Local irritation, redness, or rash.
- Management: Wash area thoroughly with soap and water. Symptomatic care.
- Store in a cool, dry place below 25°C.
- Protect from light and moisture.
- Keep out of reach of children.
TURINSE GARGLE

COMPOSITION:
| Each 5 mL contains: | |
| Benzydamine Hydrochloride BP | 7.5 mg |
| Chlorhexidine Gluconate Solution IP | |
| eq. to Chlorhexidine Gluconate | 6.0 mg |
| In pleasant flavoured aqueous base | q.s. |
KEEP ALL MEDICINES OUT OF THE REACH OF CHILDREN.
- Do not swallow the solution.
- Rinse mouth and gargle thoroughly to ensure full contact with the affected areas.
- Avoid using immediately after brushing with SLS-containing toothpaste (wait at least 30 minutes).
- May cause oral numbness or stinging (due to Benzydamine).
- Tooth/tongue staining and altered taste may occur with prolonged use (due to chlorhexidine).
- Discontinue use if irritation, swelling, or allergy develops.
- Known hypersensitivity to benzydamine, chlorhexidine, or any excipients.
- History of allergic reactions to other local antiseptics or NSAIDs.
- Toothpaste (especially SLS-based) may inactivate chlorhexidine—use the gargle at a different time.
- Avoid concurrent use with other cationic antiseptics unless advised.
- Benzydamine: Category B
- Chlorhexidine: Generally considered safe topically
- Can be used during pregnancy and breastfeeding under medical supervision; avoid prolonged use.
- Common: Mild burning, dry mouth, oral numbness, taste alteration.
- Less common: Tooth discoloration, tongue staining.
- Rare: Allergic reactions, including rash, swelling, or difficulty breathing.
- Nausea, vomiting, gastric irritation.
- CNS symptoms (very rare) like restlessness or drowsiness.
- Store at 15–25°C in a cool, dry place.
- Protect from direct sunlight and heat.
- Do not freeze.
- Keep tightly closed and out of reach of children.
ULTRANEX DT
COMPOSITION:
| Each uncoated dispersible tablet contains: | |
| Piroxicam I.P. | 20 mg |
| Excipients | q.s. |
KEEP ALL MEDICINES OUT OF THE REACH OF CHILDREN.
- Absorption: Rapidly absorbed from the gastrointestinal tract. Peak plasma concentration is reached in 3–5 hours.
- Distribution: Highly protein-bound (~99%).
- Metabolism: Metabolized in the liver, primarily via CYP2C9 enzyme.
- Elimination: Excreted mainly through the urine and faeces with a half-life of ~50 hours, allowing for once-daily dosing.
- Piroxicam 20mg Dispersible Tablets are indicated for:
- Osteoarthritis (joint pain and stiffness)
- Rheumatoid arthritis (joint inflammation and damage)
- Ankylosing spondylitis (spinal arthritis)
- Acute musculoskeletal disorders (e.g., sprains, strains)
- Postoperative pain management
General
- Use the lowest effective dose for the shortest duration to minimize side effects.
- Increases the risk of gastrointestinal (GI) ulcers, bleeding, and perforation, especially in elderly patients.
- May cause fluid retention and worsen heart failure or hypertension.
- Long-term use is associated with renal toxicity—monitor kidney function regularly.
Warnings
- GI Risk: May cause ulcers, bleeding, or perforation, especially in the elderly.
- Cardiovascular Risk: Increases risk of heart attack and stroke; avoid in CVD patients.
- Kidney Toxicity: Can cause renal failure, especially in those with CKD.
- Liver Damage: May lead to hepatitis or liver failure.
- Blood Disorders: Can cause anemia, bleeding, or thrombocytopenia.
- Fluid Retention & Hypertension: Worsens heart failure and high blood pressure.
- Allergic Reactions: Risk of anaphylaxis, Stevens-Johnson syndrome (SJS).
- Fertility Issues: May impair female ovulation.
Contraindications
- Allergy to NSAIDs or Piroxicam
- Active peptic ulcer or GI bleeding
- Severe heart disease (recent heart attack, stroke, heart failure)
- Severe kidney or liver impairment
- Bleeding disorders or anticoagulant use ∙Pregnancy (third trimester)
- Children under 12 years
Drug Interaction
- Anticoagulants (Warfarin, Heparin) - Increased bleeding risk - Monitor INR/bleeding signsAntihypertensives (ACE inhibitors, ARBs, Beta-blockers) - Reduced effectiveness - Monitor BPDiuretics (Furosemide, Thiazides) - Reduced diuretic effect - Monitor kidney functionCorticosteroids (Prednisolone, Dexamethasone) - Increased GI ulcer risk - Use gastroprotective agentsMethotrexate - Increased toxicity risk - Monitor blood levelsLithium - Increased lithium toxicity - Monitor lithium levels
- Category C (1st & 2nd trimester): Use only if benefits outweigh risks.
- Category D (3rd trimester): Contraindicated due to risk of premature closure of ductus arteriosus, delayed labor, and neonatal complications.
- Avoid use during breastfeeding as it may pass into breast milk.
Symptoms of Overdose
- Severe nausea, vomiting, and stomach pain
- Drowsiness, confusion, dizziness
- Severe GI bleeding (black stools, vomiting blood)
- Seizures, respiratory depression, kidney failure
Management
- No specific antidote—treatment is symptomatic and supportive.
- Gastric lavage & activated charcoal may help if ingestion was recent.
- Proton pump inhibitors (PPIs) or H2 blockers to protect the stomach.
- IV fluids and electrolyte correction for dehydration.
- Haemodialysis is NOT effective due to high protein binding.
- Store at room temperature (15–30°C), away from moisture and heat.
- Keep the tablets in their original packaging.
- Protect from direct sunlight.
- Keep out of reach of children.
- Do not use beyond the expiry date printed on the package.

