COFDOFF TABLET
COMPOSITION:
| Each film coated bilayered tablet contains: | |
| Acebrophylline | 100 mg |
| Acetylcysteine USP | 600 mg |
| Excipients | q.s. |
KEEP ALL MEDICINES OUT OF THE REACH OF CHILDREN.
- Acebrophylline: A xanthine derivative with bronchodilator and anti-inflammatory properties. It inhibits phosphodiesterase, increases cyclic AMP levels, and modulates inflammatory mediators. It also has mucoregulatory effects that help clear airway secretions.
- N-Acetylcysteine (NAC): A mucolytic agent that breaks disulfide bonds in mucus, reducing its viscosity and improving clearance. It also has antioxidant properties that protect against oxidative stress in chronic respiratory diseases.
- Chronic obstructive pulmonary disease (COPD)
- Bronchial asthma
- Chronic bronchitis
- Cystic fibrosis
- Respiratory tract infections with excessive mucus production
- Use with caution in patients with cardiovascular diseases, peptic ulcers, liver disorders, and kidney dysfunction.
- Avoid alcohol, as it may enhance side effects.
- Acebrophylline may cause tachycardia and should be used cautiously in patients with arrhythmia.
- N-Acetylcysteine may cause bronchospasm in sensitive individuals.
- Not recommended for patients with active gastrointestinal ulcers.
- Hypersensitivity to Acebrophylline or N-Acetylcysteine.
- Severe cardiac arrhythmias.
- Active peptic ulcer disease.
- Theophylline or other xanthines: Increased risk of toxicity.
- Corticosteroids & Diuretics: May enhance hypokalemia when combined with Acebrophylline.
- Antibiotics (e.g., Amoxicillin, Doxycycline): N-Acetylcysteine enhances the bioavailability of some antibiotics.
- Antitussives (e.g., Codeine): May reduce mucus clearance.
- Category C (Use only if benefits outweigh risks).
- Not recommended during the first trimester unless necessary.
- Common: Nausea, vomiting, heartburn, headache, dizziness.
- Rare but serious: Bronchospasm, severe allergic reactions, palpitations.
- Symptoms: Severe nausea, vomiting, tachycardia, hypotension, restlessness, seizures.
- Treatment: Symptomatic and supportive care, gastric lavage if necessary.
- Store at below 30°C in a dry place.
- Protect from moisture and direct sunlight.
- Keep out of reach of children.
COFDOFF SYRUP
COMPOSITION:
| Each 5 mL contains: | |
| Acebrophylline | 50 mg |
| Terbutaline Sulphate I.P. | 1.25mg |
| Guaiphenesin I.P. | 50 mg |
| Flavoured syrupy base | q.s. |
KEEP ALL MEDICINES OUT OF THE REACH OF CHILDREN.
- Acebrophylline: A xanthine derivative with mucoregulating and bronchodilator effects. It inhibits phosphodiesterase, increases cAMP, and modulates inflammation by inhibiting phospholipase A2.
- Terbutaline Sulphate: A β2-adrenergic receptor agonist that relaxes bronchial smooth muscle, reducing airway resistance and improving airflow.
- Guaiphenesin: An expectorant that increases respiratory tract secretions, helping to clear mucus from the airways.
- Acute and chronic bronchitis
- Chronic obstructive pulmonary disease (COPD)
- Bronchial asthma
- Respiratory tract infections with mucus build-up
- Adults: 5-10 mL (1-2 teaspoons) 2-3 times daily
- Children: (6-12 years): 5 mL (1 teaspoon) 2-3 times daily
- (2-6 years): 2.5 mL (½ teaspoon) 2-3 times daily (Or as directed by the physician)
- Use with caution in patients with cardiovascular diseases, hypertension, hyperthyroidism, and diabetes mellitus.
- Avoid alcohol as it may enhance side effects.
- Not recommended for patients with severe cardiac arrhythmias or hypersensitivity to any of the components.
- Excessive use may cause tachycardia or tremors.
- Hypersensitivity to Acebrophylline, Terbutaline, or Guaiphenesin
- Severe hypertension
- Uncontrolled cardiac arrhythmias
- Peptic ulcer disease (Acebrophylline may aggravate gastric irritation)
- Beta-blockers (e.g., Propranolol): May reduce the bronchodilatory effects of Terbutaline.
- Theophylline & other xanthines: Increased risk of toxicity when combined with Acebrophylline.
- Corticosteroids & Diuretics: May enhance hypokalemia when taken with β2-agonists like Terbutaline.
- Monoamine Oxidase Inhibitors (MAOIs) & Tricyclic Antidepressants: May increase the cardiovascular effects of Terbutaline.
- Common: Nausea, headache, dizziness, tremors, palpitations, dry mouth
- Serious: Arrhythmia, severe hypotension, hypokalemia, paradoxical bronchospasm (rare)
- Symptoms: Severe palpitations, hypertension, tremors, nausea, vomiting, restlessness, seizures.
- Treatment: Supportive care, beta-blockers (for severe tachycardia), IV fluids, electrolyte correction.
- Store at below 30°C in a cool, dry place.
- Protect from light and moisture.
- Keep out of reach of children.
COFDOFF-LS SYRUP

COMPOSITION:
| Each 5 mL contains: | |
| Levosalbutamol Sulphate IP | |
| eq. to Levosalbutamol | 1 mg |
| Ambroxol Hydrochloride IP | 30 mg |
| Guaiphenesin IP | 50 mg |
| Mentholated flavoured syrupy base | q.s. |
KEEP ALL MEDICINES OUT OF THE REACH OF CHILDREN.
- Levosalbutamol (1mg): A β2-adrenergic agonist that relaxes airway muscles, reduces bronchospasms, and improves airflow.
- Ambroxol (30mg): A mucolytic agent that breaks down thick mucus, facilitating its removal from the respiratory tract.
- Guaiphenesin (50mg): An expectorant that loosens and thins mucus, making coughing more productive.
- Levosalbutamol: Absorbed within 30 minutes, peak effect at 2 hours, half-life 3–5 hours, excreted via urine.
- Ambroxol: Rapid absorption, onset within 30 minutes, half-life 10 hours, eliminated primarily in urine.
- Guaiphenesin: Rapid absorption, short half-life 1 hour, excreted via urine.
- Acute and chronic bronchitis
- Asthma-associated cough
- Chronic obstructive pulmonary disease (COPD)
- Pneumonia and lower respiratory infections
- Cough with thick mucus production
- Adults: 5–10 mL (1–2 teaspoons) three times daily
- Children (above 12 years): 5–10 mL (1–2 teaspoons) three times daily
- (6 – 12 years): 5 mL (1 teaspoon) three times daily
- (2 – 6 years): 2.5 mL (½ teaspoon) three times daily
- Infants (Below 2 years): Use only under medical supervision. 1.25–2.5 mL two to three times daily (as prescribed by a doctor)
- Use with caution in patients with heart disease, high blood pressure, hyperthyroidism, or diabetes.
- Avoid use in patients with a history of gastric ulcers or GI bleeding, as Ambroxol may cause irritation.
- May cause drowsiness or dizziness; avoid driving or operating heavy machinery.
- Hypersensitivity to Levosalbutamol, Ambroxol, or Guaiphenesin.
- Severe heart diseases such as arrhythmias or recent myocardial infarction.
- Peptic ulcer disease or GI bleeding history (due to Ambroxol).
- Uncontrolled hypertension or hyperthyroidism (due to Levosalbutamol).
- Renal or hepatic impairment (use with caution and under medical supervision).
- Beta-blockers (Propranolol, Atenolol) - Antagonizes Levosalbutamol - Reduced bronchodilation
- Diuretics (Furosemide, Thiazides) - Increased risk of hypokalemia - Monitor potassium levels
- CNS Depressants (Alcohol, Sedatives, Antihistamines) - Increased drowsiness - Avoid alcohol
- Antibiotics (Erythromycin, Ciprofloxacin) - Increased Ambroxol absorption - Monitor for side effects
- Cough Suppressants (Codeine, Dextromethorphan) - Opposes Guaiphenesin action - Reduced mucus clearance
- Pregnancy: Should be used only, if necessary, particularly in the first trimester.
- Lactation: Ambroxol and Levosalbutamol may pass into breast milk, so use with caution.
- Gastrointestinal - Nausea, vomiting, diarrhoea - Peptic ulcer, GI bleeding
- Cardiovascular - Palpitations, mild tachycardia - Arrhythmia, severe hypertension
- Respiratory - Throat irritation, dry mouth - Paradoxical bronchospasm
- CNS - Dizziness, headache - Seizures, tremors
- Allergic Reactions - Skin rash, itching - Anaphylaxis, angioedema
- Levosalbutamol Overdose: Severe palpitations, tremors, muscle cramps, hypokalemia.
- Ambroxol Overdose: Excessive nausea, vomiting, diarrhoea.
- Guaiphenesin Overdose: Dizziness, confusion, respiratory depression.
- Symptomatic and supportive treatment is required.
- Gastric lavage & activated charcoal in early overdose cases.
- Beta-blockers (e.g., propranolol) for severe Levosalbutamol-induced tachycardia.
- IV fluids & electrolyte correction if needed.
- Store at room temperature (15–30°C).
- Keep in a cool, dry place, away from direct sunlight.
- Do not refrigerate or freeze.
- Keep out of reach of children.
- Use before the expiry date mentioned on the bottle.
COFDOFF-D SYRUP
COMPOSITION:
| Each 5 mL contains: | |
| Chlorpheniramine Maleate USP | 2 mg |
| Dextromethorphan Hydrobromide USP | 10 mg |
| Phenylephrine Hydrochloride BP | 5 mg |
| In a pleasantly flavoured syrup | q.s. |
KEEP ALL MEDICINES OUT OF THE REACH OF CHILDREN.
Cough is a protective respiratory reflex by which foreign matter is expelled from the tracheobronchial tree. When cough increases in frequency and severity, it becomes troublesome, disturbs sleep and requires symptomatic treatment. Cough can be caused by disorders of upper and lower respiratory tract. Most coughs associated with acute respiratory infections or allergy or nonproductive or dry and irritating. COFDOFF-D is formulated to provide symptomatic relief of cough and upper respiratory symptoms associated with allergy or common cold, including nasal congestion.
Chlorpheniramine is a potent antiallergic agent which inhibits the vascular response to histamine thereby reducing irritation of cough receptors lining the respiratory mucous membrane. It provides relief from symptoms of allergic upper respiratory catarrh. Chlorpheniramine has moderate sedative and antimuscarinic effects. After absorption, it is widely distributed in body and extensively metabolized; unchanged drug and metabolites are excreted primarily in urine.
Dextromethorphan Hydrobromide is a nonopioid cough suppressant. Dextromethorphan suppresses the cough reflex by a direct action on the medullary cough centre. Dextromethorphan is as effective as codeine in antitussive effect. However, it does not have addictive, sedative or respiratory depressant effect seen with opioid antitussives. The onset of antitussive action is 15-30 minutes after administration and is sustained for upto 6 hours. Dextromethorphan is rapidly absorbed after oral administration. It is metabolized in liver and excreted in urine as unchanged drug and metabolites.
Phenylephrine Hydrochloride is a predominantly indirect acting sympathomimetic agent with a decongestant action on nasal and upper respiratory tract mucosal membranes. Phenylephrine causes far less central nervous system stimulation than ephedrine. The onset of decongestant action is 15-30 minutes, peak activity occurring after approximately one hour. Phenylephrine is readily and completely absorbed after oral administration. It is excreted largely unchanged in urine.
COFDOFF-D cough formula is indicated for symptomatic relief of nonproductive dry cough and upper respiratory symptoms such as irritation of throat, running nose, nasal congestion and watery eyes associated with allergy or common cold. It may also be useful in coughs associated with upper respiratory tract infection.
- Adults: 10ml (2 teaspoonfuls) 3-4 times a day.
- Children: 6-12 years : 5ml (1 teaspoonful) 3-4 times a day.
- 2-6 years : 2.5ml (1/2 teaspoonful) 3-4 times a day or as directed by the physician
ELYRIA-M BILAYERED TABLET
COMPOSITION:
| Each film coated bilayered tablet contains: | |
| Bilastine IP | 20 mg |
| Montelukast Sodium IP | |
| eq. to Montelukast | 10 mg |
KEEP ALL MEDICINES OUT OF THE REACH OF CHILDREN.
- Allergic rhinitis (seasonal and perennial)
- Chronic urticaria
- Asthma (mild to moderate) – as add-on therapy
- Allergy-related respiratory symptoms (nasal congestion, sneezing, rhinorrhoea, wheezing)
- Avoid alcohol and sedatives during treatment
- Montelukast is not meant for acute asthma attacks
- Bilastine has minimal CNS effects but caution in patients with sensitivity to antihistamines
- Rare reports of neuropsychiatric events (agitation, anxiety, depression, suicidal thoughts) associated with Montelukast
- Use caution in patients with hepatic impairment
- Do not exceed recommended dosage
- Known hypersensitivity to Bilastine, Montelukast, or any excipients ∙Patients with rare hereditary galactose intolerance (if excipients contain lactose)
Drug Interaction
- Bilastine: Absorption may be reduced with grapefruit juice or other acidic beverages
- Use with phenobarbital or rifampicin may reduce plasma levels
- No significant interaction with theophylline, warfarin, digoxin, or oral contraceptives
- Common: Headache, Drowsiness (low incidence), Abdominal pain and dry mouth.
- Less common / rare: Behavioural changes (irritability, insomnia, agitation), Skin rash, Elevated liver enzymes (rare) and Anaphylaxis (very rare)
- Store in a cool, dry place, below 25°C
- Protect from moisture and light
- Keep out of reach of children
NASODOFF S SPRAY
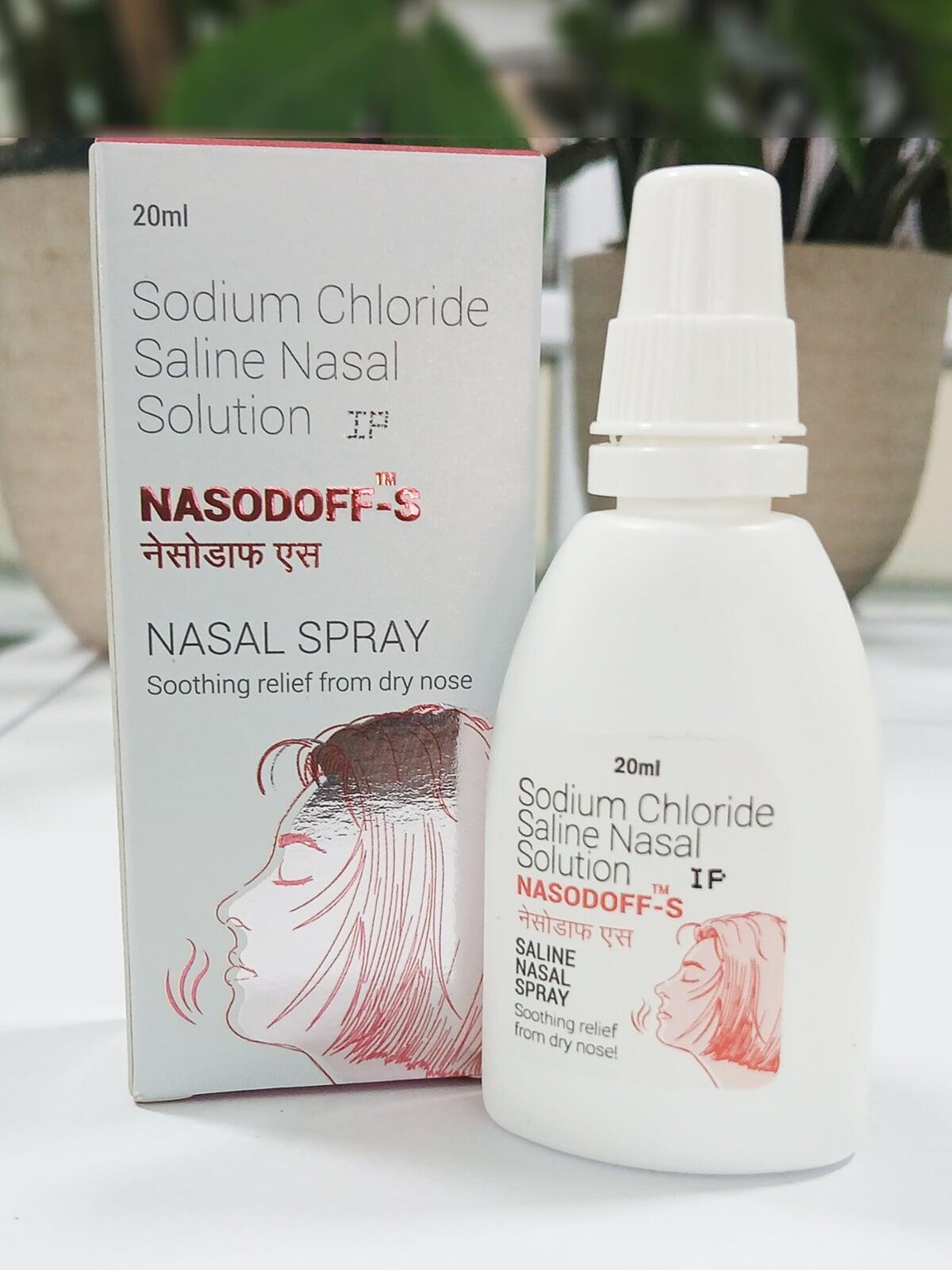
COMPOSITION:
| Each mL contains: | |
| Sodium Chloride IP | 0.74% w/v |
| Purified water IP | q.s. |
KEEP ALL MEDICINES OUT OF THE REACH OF CHILDREN.
- Nasal congestion and dryness
- Allergic rhinitis or sinusitis
- Soothing irritated nasal passages (due to pollution, dry air, or infections)
- Daily nasal hygiene
- Post-nasal surgery care
- Assisting in clearing thick mucus in infants, children, and adults
- For nasal use only
- Do not share the nozzle with others to avoid contamination
- Use under medical advice in infants under 6 months
- Do not use if the solution is cloudy or past expiry date
- In case of persistent symptoms, consult a physician
- Hypersensitivity or known allergy to Sodium Chloride (very rare)
- Do not use in case of severe nasal injury or active bleeding without medical supervision
- No known drug interactions ∙Safe to use with other nasal medications (can be used before medicated sprays for better absorption)
- Safe for use during pregnancy and breastfeeding
- Non-medicated and non-systemic
- Overdose is unlikely due to the non-systemic nature of the product
- Excessive use may cause nasal dryness or irritation
- Store at room temperature below 25°C
- Keep away from direct sunlight and moisture
- Keep out of reach of children
- Do not use if packaging is damaged

